- Amino acid compositions
- with blind values
- without blind values
- of resin bond
We offer one of the most efficient amino acid analysis,the Enantiomerlabeling: The amino acid enantiomers are used as excellent multi-internal standards.
For the calculation the enantiomeric purity of the standard as well of the sample is taken into account.
Calculation of the amino acid concentration:
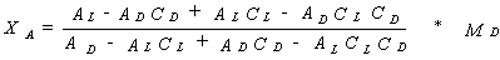
AL = peak area of L enantiomer after addition of standard
AD = peak area of D enantiomer after addition of standard
CL = D enantiomer : L enantiomer of the sample
CD = L enantiomer : D enantiomer of the standard amino acid
MD = amount of standard amino acid added
XA = amount of amino acid being determined
Two methods are available:
with determination of blind values:
The enantiomeric purity of the amino acids after! hydrolysis is determined.
without determination of blind values:
the peptide is assumed to have the correct configuration for all amino acids and no contaminants by the wrong enantiomers. Furthermore it is assumed that
no substance specific racemization take place during hydrolysis. Therefore this method is not capable for GMP-products.
- For more details download PDF Publication.
- For more details download PDF Introduction.
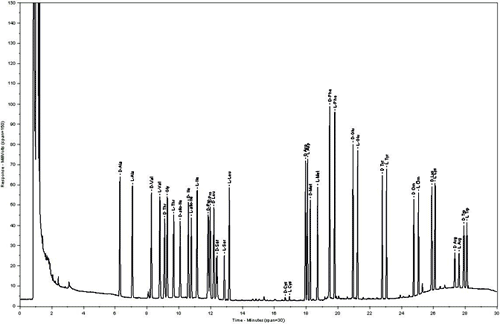
Not only the quantitative composition, also the optical purity of the amino acids are determined. The quantitative amino acid analysis is done by Enantiomerlabeling: By capillary gas chromatography on a chiral stationary phase it is possible to separate the amino acids together with their enantiomers, thus allows the determination of enantiomeric purity after hydrolysis. In a second analytical run the sample is spiked with an aliquot of the optical antipodes of the amino acids and d-norvaline as standard for glycine. These serve as ideal multi internal standards, which are calibrated against certified standards from National Institute of Standards and Technology (NIST).
Results: Enantiomeric purity after! Hydrolysis ( = blind values ) Content of amino acids (mg/g, umol/g) Residues of amino acids Peptide content.
- For description of the method please contact info@cat-online.com
If the peptide is known to be optical pure the determination of optical purity will be skipped and is set to 100%. The method is not useful for GMP-peptides due to the potential error caused by unexpected racemization of the peptide either during hydrolysis or synthesis.
Results: Content of amino acids (mg/g, µmol/g) Residues of amino acids Peptide content

- For information about choice of method please contact info@cat-online.com
Quantitative determination of the amino acid which is loaded at the resin gives the information of the capacity of the resin.
Results: Content of amino acid (mg/g, µmol/g)

- For description of the method please contact info@cat-online.com